Our recommendations are made independently. We may receive commissions from purchases made via our links.
Hard Water Vs Soft Water: All You Need to Know
Hard water is a problem in many parts of the country. Here is all of the information you need to know about the debate between hard water vs soft water.
It’s normal to think of the water dripping out of the house’s tap as just that— water— without any other special quality to it. In reality, this is far from the truth. The water flowing through your pipelines will arrive in one of two states: either as hard water or soft water.

Knowing the difference between them (and taking corrective action, if need be) can make a highly positive impact on your family’s health and overall water safety.
In this hard water vs soft water guide, we will look into each type of water and their respective advantages and disadvantages. You will also learn about the different methods used to control the hardness of household water.
Hard Water vs Soft Water: What Does it Mean?
Defining Hard & Soft Water
When we speak about water’s hardness, we are referring to the mineral content of the water. The more minerals (calcium, magnesium, carbonates, bicarbonates, etc.) there are in the water, the harder the water.
So with this in mind, hard water is simply water that contains a high volume of dissolved minerals. Meanwhile, soft water is the opposite, having a lower concentration of dissolved minerals.
But though there can be many types of minerals in your water stream, most water hardness tests only measure calcium carbonate.
There are two ways that calcium carbonate concentration is measured: Grains of hardness Per Gallon (GPG) or Parts Per Million (PPM).
PPM can also be expressed as milligrams per liter (mg/l).
1 GPG unit is equal to 17 PPM (17 mg/l).
You can use this table as a simple reference.
Hardness Level | PPM (mg/l) | GPG |
|---|---|---|
Soft | Less than 17 mg/l | Beneath 1.0 |
Slightly Hard | 17 - 60 mg/l | 1.0 - 3.0 |
Moderately Hard | 61 - 120 mg/l | 3.5 - 7.0 |
Hard | 121 - 180 mg/l | 7.0 - 10.5 |
Very Hard | Greater than 180 mg/l | Greater than 10.5 |
How Hard Water Forms
Water is one of the best solvents out there so it can readily pick up impurities. But water from most sources also contains a very weak carbonic acid as a result of its interaction with atmospheric carbon dioxide. The carbonic acid makes groundwater an even better solvent, allowing it to take in more minerals and become harder than it could otherwise be.
When groundwater moves across layers of permeable soil and rocks (as occurs in aquifers), it also picks up a lot of minerals and other compounds in the process via a process called percolation.
Calcium and magnesium are plentiful within aquifers and are some of the most water-soluble minerals out there. Thus, when groundwater rushes across underground deposits of these minerals (limestone, gypsum, chalk, etc.), it picks them up and the result is hard, mineral-laden water.
Hard water can be caused by other elements, too, like iron and manganese. They can be picked up via the same process (passing through iron and manganese ores or rock layers with high concentrations of these metals).
Who Should Be Worried About Hard Water?
If you use municipal water (city water), you typically don’t have to deal with water hardness issues often. After going through numerous filtration steps and chlorination, the water that arrives at your taps from your local water treatment plant will have been softened considerably.
That doesn’t mean hard water is never an issue for those using public water, however. It’s just not the norm. If you do have hardness-related problems, inform the water department — they should resolve it for you.
Private wells, on the other hand, are where the majority of problems with water hardness are reported. Many wells in the United States (especially in the east-central and western regions) draw water from aquifers with high levels of dissolved minerals.
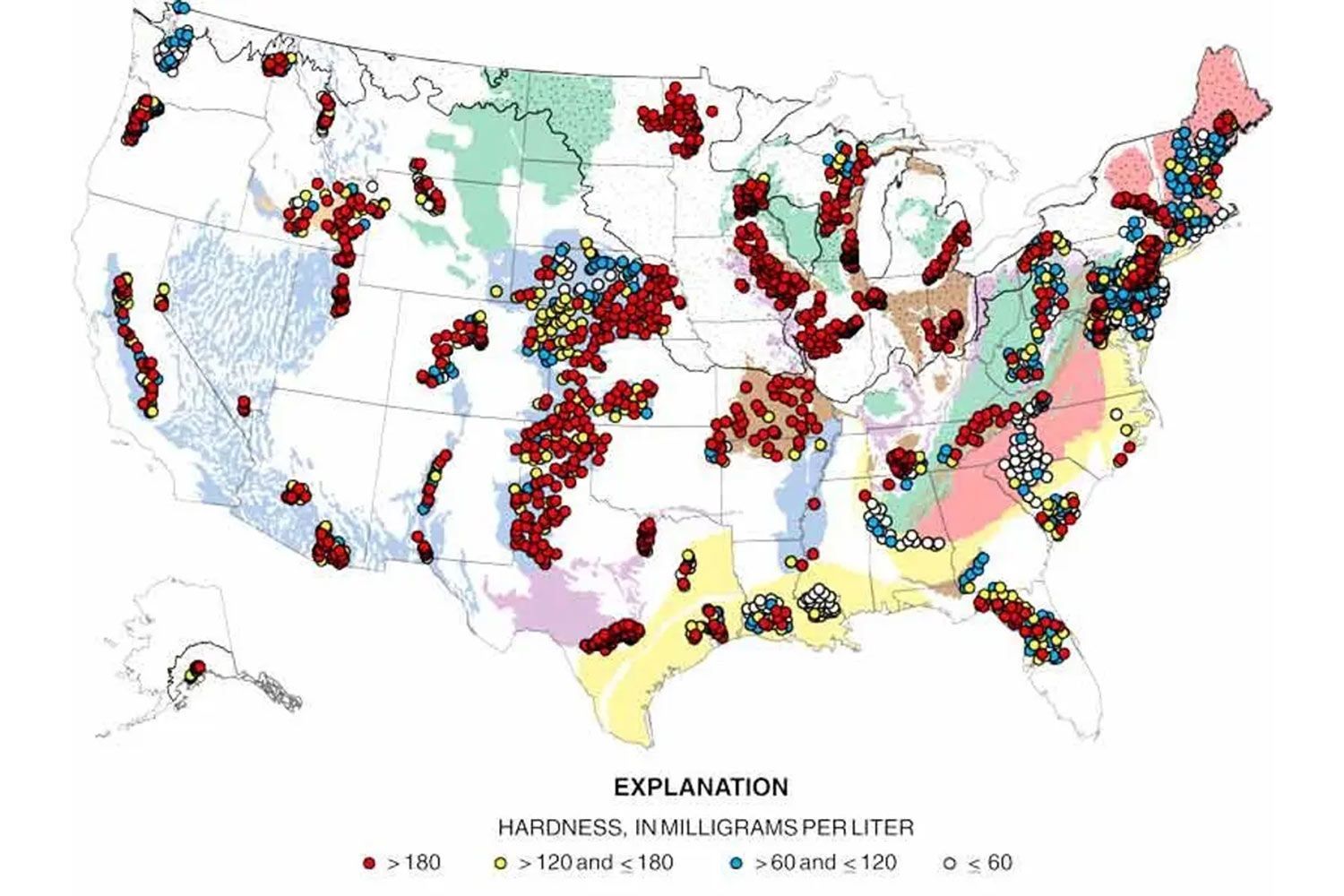
Carbonate aquifers, for example, are widespread in the eastern U.S. This kind of aquifer consists of limestone, dolomite (a carbonate mineral made up of calcium and magnesium), and marble. The limestone and dolomite deposits can bleed a lot of their contents into the water, causing the water’s hardness to reach disconcertingly high levels.
According to the survey chart above, most domestic wells located in areas known for carbonate aquifers rate in the “very hard” range (above 180 mg/l).
Iron/manganese-laden hard water can be found in deep wells, where the water has a longer contact time with the rock and soil layers. It can also be the result of mining activities, so if you’re in a mining region, you run a higher risk of encountering iron/manganese-infested water.
Hard Water vs Soft Water: How to Tell?
You don’t need full-fledged scientific instruments or complicated water tests to tell if your house is running on hard water. The effects of hard water can be observed with an act as simple as washing your hands using common soap.
If the water is too hard, you may see what’s known as soap scum on your hand. This sticky, slimy film of precipitation is the result of a chemical reaction between soap and the calcium in the water.
You can also get the same unsightly result while washing drinking glasses, too. Ever noticed white, blurry spots or films on the glasses after your freshly washed glasses dry? That’s calcium precipitation and is a symptom of hard water.

A quicker, more direct water hardness test is to simply boil some tap water. Calcium carbonate solidifies at higher temperatures, so you can spot solid deposits and milky white chunks of calcium at the bottom of the pot.

You should be able to tell iron/manganese hard water apart from others by the color as well as the distinct metallic taste.
The water will initially come out of the tap showing no coloration, but when left exposed to the air (and thus, in contact with oxygen) for some time, the color can change. If the water has a high concentration of iron, it can adopt an orange-brownish tint.

Water with high manganese content will turn black.

Drinking Soft Water vs Hard Water: Which is Better?
Academia has long debated whether or not hard water is harmful to drink. Fortunately, most of the research done so far suggests that no, hard water won’t negatively affect your health if you drink it.
Surprisingly, some research also points to a few potential health benefits of drinking hard water, as well.
Hard water, as we mentioned earlier, typically contains a lot of calcium and magnesium. It turns out that both of these are essential minerals for the human body. The pair is particularly important for bone development and health. A serious deficiency of either (or both) can lead to osteoporosis, a disease characterized by weak, brittle bones that can fracture more easily.
The same thing also applies to iron/manganese hard water: a little bit of both are healthy. Iron is a crucial component in blood cells and manganese participates in bone development, among other roles it has in the body’s function.
As a result, hard water can be a good supplement for anyone whose diet is lacking in these minerals.
There have also been a few studies that linked consuming hard water with minor improvements in cardiovascular health. However, so far the evidence for this claim hasn’t shown itself to be conclusive, so take this piece of info with a grain of salt.
Why You Should Watch Out For Hard Water
As expected, hard water is not all good. It’s probably better than soft water for drinking, yes, but for household tasks like showering or laundering, hard water is a nuisance.
It Can Damage Your Plumbing and Shower
Excessively hard water can wear down household appliances that use water in large amounts. Showerheads, for example, can quickly accumulate tough calcium deposits after some time. If you don’t clean these deposits, they can clog the showerhead and reduce water pressure or water flow.
The same thing goes for water pipes. The minerals deposited over time on the pipe walls can build up like arterial plaques, gradually clogging the system. The harder the water, the quicker those deposits can build up in your pipes. This will leave you spending more money on pipe maintenance and even replacement as a result.

Skin Issues
Hard water doesn’t just damage your shower or plumbing system; showering in it can also give you skin problems. Calcium salts can cause a dry and itchy scalp if it accumulates on your head and in your hair. You may also notice heavier dandruff if your water is too hard.
Showering and soaping under particularly hard water can leave a layer of slimy soap scum on your skin. It can suck out moisture from your pores and leave you itchy and uncomfortable instead of refreshed like a shower should.
Additionally, hard water can raise the pH level of your skin’s surface. Human skin is normally slightly acidic while hard water is alkaline. Showering in hard water can raise the pH level of your skin and disrupt its protective abilities. Bacteria can then build up on your skin and cause problems like atropic dermatitis (eczema).
Related post: How to Make Alkaline Water: Six Simple Ways
Damage to Your Clothes
If your washing machine uses hard water, the minerals can build up on your clothes in the form of white stains. But they don’t just make your clothes look soiled and unappealing, the tough mineral coatings can also weaken the fibers, making them more prone to rips and tears.

Stains on Appliances
Hard water that’s high in iron and manganese can cause unsightly stains on appliances and fixtures like sinks, toilets, and washing machines. High-iron water causes orange-brown stains while high-manganese water paints your fixtures with hideous black streaks.

What Is Water Softening?
To combat hard water, water softening systems are employed.
Such systems are designed to filter out all of the hardness-causing particles like calcium, magnesium, and other metallic ions. When all of these minerals are removed, your water supply will become much softer and more usable for showering and other water tasks around the house.
How Water Softening Systems Work
Household water softening systems typically use an ion-exchange medium to remove undesired elements. This medium can either be made of zeolites or synthetic resin beads. According to the NSF/ANSI Standard 44, the job of an ion-exchange medium is to: “reduce hardness caused by calcium and magnesium ions and [replace] them with sodium or potassium ions.”
In other words, when hard water— bloated with calcium, magnesium, and other metallic ions— flows through the medium, the “hardening” minerals are absorbed and replaced by either sodium or potassium ions (depending on the exchange solution).
Sodium and potassium do not cause hardness. As a result, their presence in the water will not yield the same irritating effects as calcium, magnesium, and other metallic ions. And before you get worried, sodium and potassium are non-toxic as well, so their presence in the water will not cause any health issues when used or consumed. In fact, they’re also essential minerals that must be included in your diet.
The majority of water softeners on the market utilize sodium as an exchange ion rather than potassium. This is because salt brine— the solution that’s used for the sodium-based ion-exchange process— is cheap and easy to buy. You only need to fill up the brine tank with normal table salt and brine will be available immediately for use.
A 40-pound bag of salt costs about $5 to $8.
Meanwhile, to use potassium, you must fill the tank with potassium chloride. A 40-pound bag of this stuff will cost you upwards of $25.
As for how efficient these systems are, you shouldn’t have cause for worry. The majority of the models on the market today can eliminate nearly all traces of calcium and magnesium from the water.
The ion-exchange action mainly removes calcium and magnesium. Fortunately, most water softening systems are also capable of filtering iron and manganese (as much as 10 PPM).
What is the Regeneration/Recharge Process?
For the softener to keep on working properly, trapped minerals and debris must be cleansed from the system after two or three days. This process is formally known as regeneration (it can also be called “recharge”).
How Long is the Regeneration Process?
It should take the machine about two hours to complete a regeneration cycle.
While it is regenerating, the softener is put into bypass mode. In this mode, the water that is delivered to your house will be unprocessed, hard water. That’s the reason why manufacturers recommend that you do not use water during this time.
The Regeneration Cycles
The regeneration process usually consists of five cycles.
Fill Cycle
The first cycle includes filling the brine tank with water. There should already be dry salt inside the brine tank beforehand. Thus, when water flows in, a salty brine solution is created.
This brine is an essential cleaning agent for the softener that will help remove all of the accumulated minerals.
Brine Draw Cycle
In this cycle, the newly-generated brine is drawn out of the brine tank, through the softener, and across the resin-filled medium. As the brine flows across the saturated beads, it forces out all of the precipitated minerals that have built up. Those loosened minerals can then be carried out to the drain along with the brine.
The brine draw cycle usually takes about an hour and is considered complete when all of the brine has been completely drained from the softener.
Slow Rinse Cycle
Once the brine has been drained, the softener draws in pressurized fresh water from the inlet pipe. The job of this stage is to purge any remaining brine from the system and clear away any debris that is still stuck within.
Backwash Cycle
During the backwash cycle, water is forced backward through the softener unit and out the softener's drain. This powerful backwash can shake loose residual sediment, dirt, and debris stuck on the medium, blasting it out the drain.
On average, this step takes 15 minutes to complete.
Fast Rinse Cycle
The backwash cycle is immediately followed by the fast rinse cycle. Like the slow rinse cycle, its primary job is to flush out any leftover brine, sediment, and debris that still linger inside the equipment.
But this cycle has another purpose as well. It puts pressure on the resin beads and compacts them together.
This final cycle can take 15 minutes. Once it’s completed, the softener will revert back to normal service.
Types of Water Softening Systems
All water softeners operate for the same basic purpose: hard water in, soft water out. Their differences lay in how they regenerate.
Semi-Automatic Water Softener
The first type is the semi-automatic water softener. With this type, you have to manually start the regeneration process yourself by pushing a button. Once initiated, the softener will do all of the flushing work by itself.
Automatic Water Softener
If you don’t want to bother with this, get the second type: an automatic water softener. This is the most popular type out there.
Your automatic water softener will have a timer that you can manually set up. At the preset time, the regenerating cycle will begin and the softener will complete the process on its own.
Demand-Initiated Regeneration (DIR) Softener
This is the most advanced and expensive type of softener you can possibly buy. Such a system usually consists of three different tanks: two softening tanks and one brine tank. While one softening tank is being regenerated, the other tank will kick in to provide you with uninterrupted soft water.
The first time you set up your DIR softener, you will have to set its system for your water’s expected hardness level (it must be professionally measured). Once you do, the onboard microprocessor will calculate exactly how much water it can process before the resin beads become saturated by minerals. The cut-off limit is typically between 70% and 80% saturation.
During day-to-day operation, when the softener has reached its calculated limit, it will automatically set up a regeneration session for that night at around 2 A.M to 4 A.M. The rationale is that between 2 A.M and 4 A.M users are least likely to need water.
Off-Site Regeneration (OSR) Softener
This type isn’t popular due to its cost and complexity. An Off-Site Regeneration (OSR) system has a removable softening tank that, once saturated, must be replaced by a new, regenerated tank. The spent tank would be shipped off to a central location to be renewed.
Conclusion
Hard water can be annoying, but once again, it’s not inherently harmful to drink. Indeed, it can even be somewhat beneficial to your health.
However, if excessive water hardness is an issue in your household, the best way to tackle the problem is to install a water softening system. Purchase your new system and have it professionally installed by your local plumber. We hope that this hard water vs soft water guide has given you enough information to get you started and to anticipate how to use your new softener once it’s in place.